Pipeline
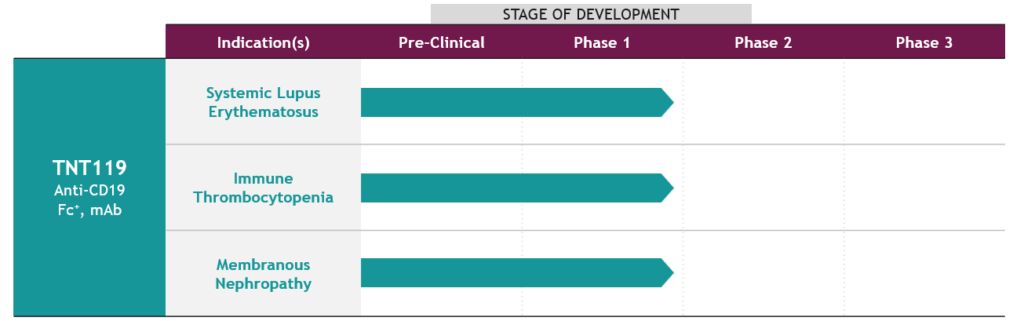
| Product Candidates (Mechanism) | Programs | Preclinical | Phase 1 | Phase 2 | Phase 3 |
| ETX-155 (GABAA receptor PAM) |
Major depressive disorder (MDD)
Focal onset seizure (FOS)
|
|
|||
|
Kv7 Program(Kv7.2/3 potassium channel opener)
|
Pain, Epilepsy, Depression
|
|
|||
Product
Candidates
(Mechanism)
Candidates
(Mechanism)
Kv7 Program
(Kv 7 .2/3 potassium
channel opener)
(Kv 7 .2/3 potassium
channel opener)
Programs
Major
depressive
disorder
(MDD)
depressive
disorder
(MDD)
Focal
onset
seizure
(FOS)
onset
seizure
(FOS)
Pain
Epilepsy
Depression
Epilepsy
Depression
Preclinical
Phase 1
Phase 2
Phase 3
Contact Us
MEDIA
INVESTOR RELATIONS
HUMAN RESOURCES
PARTNERING
Eliem Therapeutics, Inc.
23515 NE Novelty Hill Road, Suite B221 #125
Redmond, WA 98053
Eliem Therapeutics (UK) Ltd: Reg. 11893311
3rd Floor, 1 Ashley Road
Altrincham, Cheshire WA14 2DT
23515 NE Novelty Hill Road, Suite B221 #125
Redmond, WA 98053
Eliem Therapeutics (UK) Ltd: Reg. 11893311
3rd Floor, 1 Ashley Road
Altrincham, Cheshire WA14 2DT
